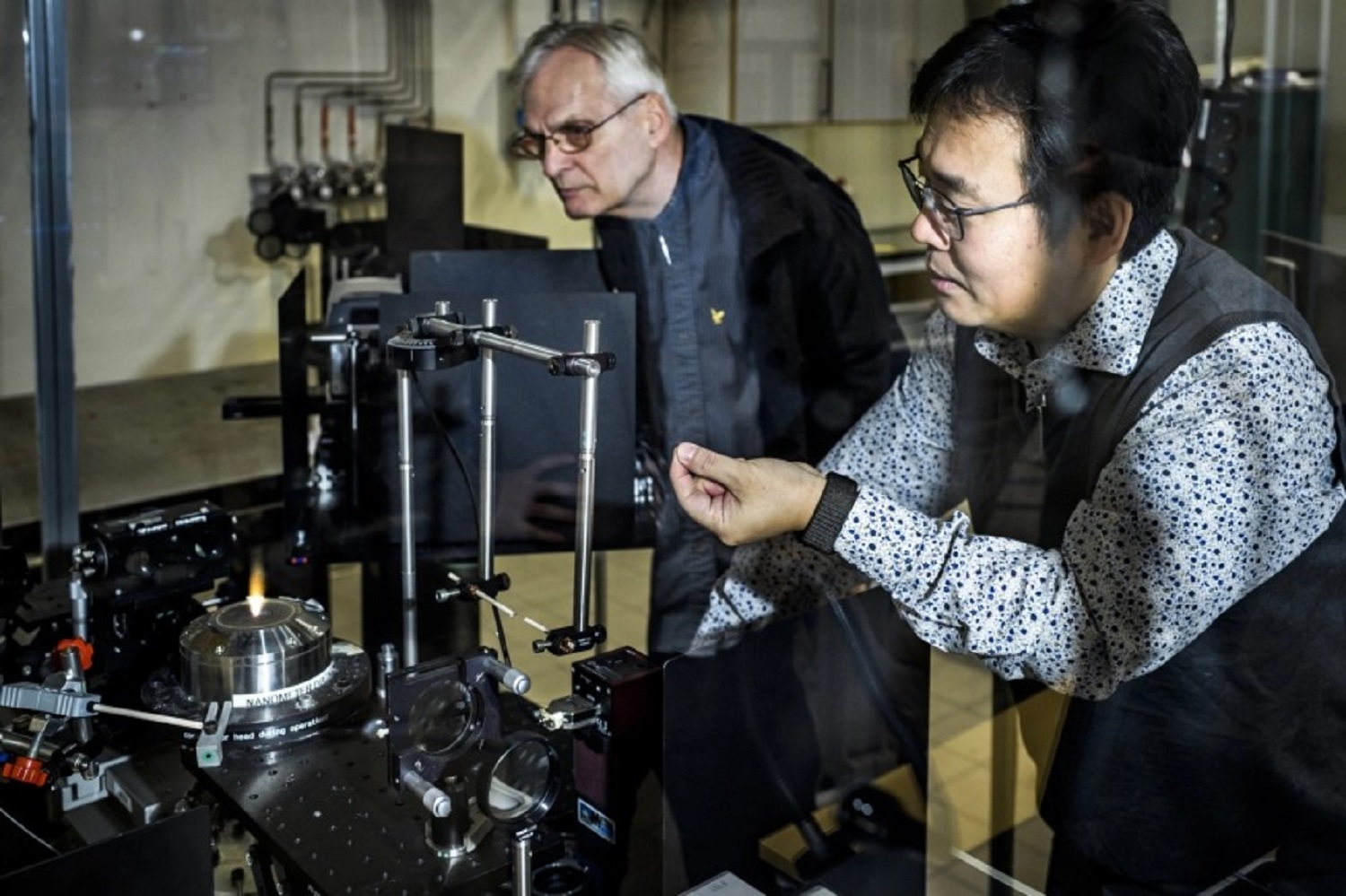
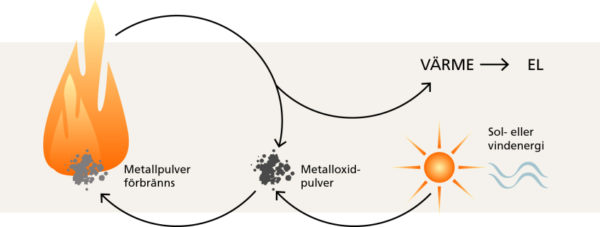
In practice, this would mean using excess energy from, for example, photovoltaics to burn metal to generate heat. Iron and aluminum can be used for this. It can then interact with hot air or steam.
Read also: Rare earth metals are not rare. This is good news for the green transition
Under these conditions, heat is released that can be used, for example, to power a turbine. As a result of combustion, a metal oxide is also formed in the form of a powder. Most importantly, again with “green” energy, said powder can be transformed back into the classic form of the mineral. All this without using fossil fuels and at a low cost.
Burning metal causes it to take on a powder form that can restore it to its original state
How to re-oxide the metal? This can be done by electrolysis, for example by mixing the powder with cryolite in which two conductive electrodes are inserted. Under these conditions, the desired chemical reaction begins.
Together with some research groups in Germany, Canada and the Netherlands, we have concluded that these common minerals are so promising as an energy source and energy carrier that they can act as an energy supply component.
Idea authors conclude
Read also: Poland does not deal well with renewables. Giving up combustion cars is even more ridiculous
Storing energy from renewable sources is one of the biggest challenges in the context of the green transition. Currently, all types of batteries are especially popular, but most often they are based on lithium – an element with a shrinking resource, the operation of which is associated with huge environmental costs.

Echo Richards embodies a personality that is a delightful contradiction: a humble musicaholic who never brags about her expansive knowledge of both classic and contemporary tunes. Infuriatingly modest, one would never know from a mere conversation how deeply entrenched she is in the world of music. This passion seamlessly translates into her problem-solving skills, with Echo often drawing inspiration from melodies and rhythms. A voracious reader, she dives deep into literature, using stories to influence her own hardcore writing. Her spirited advocacy for alcohol isn’t about mere indulgence, but about celebrating life’s poignant moments.