Chalmers University of Technology representatives write about their achievements in: Journal of the Electrochemical SocietyAs they explain, thanks to the new galvanizing strategy, they were able to make metal batteries safer to use and have higher operational stability. This in turn will translate into an extension of their service life, which has so far been a serious problem for this technology.
Read also: This will be the first solar power plant of its kind in the world. The performance will speak for itself.
Energy storage is currently a very important issue. Batteries are used in both small electronic devices and larger cars. Electric cars are becoming increasingly popular, so the demand for these batteries will certainly not decrease in the coming years. However, there are other applications, such as harvesting energy from renewable sources.
The conclusion is clear: engineers still need to work on innovative designs that can replace the current mainstream lithium-ion batteries. Their metal counterparts have excellent potential for implementation in the automotive industry. Electric cars equipped with such devices could gain a greater range thanks to the favorable weight-to-capacity ratio of metal batteries.
Metal batteries are an attractive alternative to lithium-ion batteries, among other things, due to their high storage capacity.
Their name comes from the use of electrodes made of metal. Solid electrolyte batteries can be an example of this, although in their case they were complicated by the fact that the metal they contain is highly reactive. As a result, it reacts with the environment, which is not always welcome from a chemist’s point of view. This certainly does not work in the case of energy storage devices.
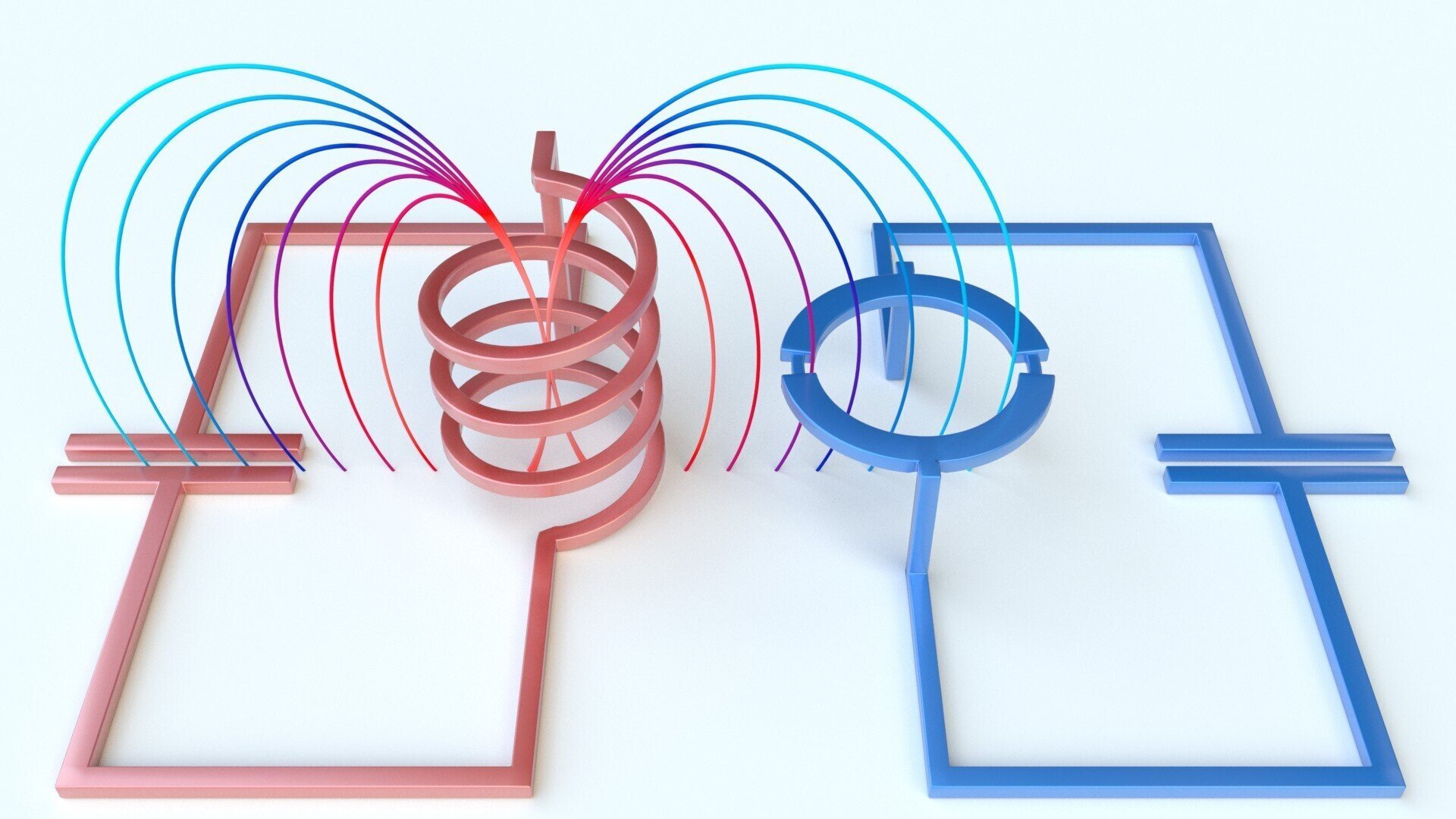
As Swedish researchers have noted, lithium metal batteries can produce so-called dendrites. They are formed during charging and discharging of batteries, and their impact on the operation of these devices is certainly negative. Dendrites disrupt operational stability and shorten battery life. During the experiments, representatives of Chalmers University of Technology realized that there is a way to combat the problem.
Read also: A battery that can stretch impressively has been created in China. The flexible battery actually works
They did this by creating an electrode inside the battery through a process known as electroplating. The metal is not susceptible to reactions with the environment, reducing the risk of dendrites forming. How does galvanization work? This process involves transferring electrons to the electrode, after which metal is formed on the surface of the electrode as a result of the electrons interacting with ions from the electrolyte. Using a similar principle, you can create a metal electrode directly inside the battery cell.

Echo Richards embodies a personality that is a delightful contradiction: a humble musicaholic who never brags about her expansive knowledge of both classic and contemporary tunes. Infuriatingly modest, one would never know from a mere conversation how deeply entrenched she is in the world of music. This passion seamlessly translates into her problem-solving skills, with Echo often drawing inspiration from melodies and rhythms. A voracious reader, she dives deep into literature, using stories to influence her own hardcore writing. Her spirited advocacy for alcohol isn’t about mere indulgence, but about celebrating life’s poignant moments.